

Sugunesh Sivalingam
6 Minutes read
Lay Summary – A Layman summary about Study trial for Common People
Clinical trials are crucial in determining the safety and effectiveness of new medical treatments and interventions. However, the technical and scientific jargon used in these trials can be complicated for the general public to understand. Lay summaries aim to bridge this gap by presenting trial results in plain language that is easy to comprehend. These summaries make clinical trial findings accessible and transparent to patients, caregivers, and the public. In this blog, we will explore the importance of lay summaries in the context of clinical trials and how they contribute to improving patient care and treatment options. Further, we will explore the additional information that lay summaries may provide and how it can aid in understanding the study results and their implications. Have a look at the storyboard below:
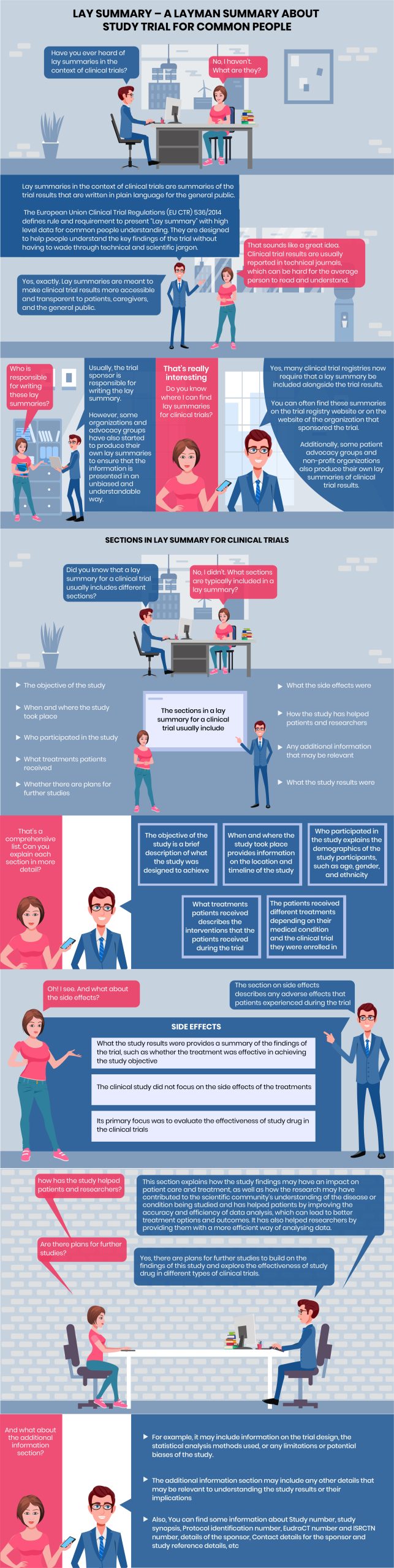
Infographic Content
Statistical Programmer: Have you ever heard of lay summaries in the context of clinical trials?
Common Man: No, I haven’t. What are they?
Statistical Programmer: Lay summaries in the context of clinical trials are summaries of the trial results that are written in plain language for the general public.
The European Union Clinical Trial Regulations (EU CTR) 536/2014 defines rule and requirement to present “Lay summary” with high level data for common people understanding. They are designed to help people understand the key findings of the trial without having to wade through technical and scientific jargon.
Common Man: That sounds like a great idea. Clinical trial results are usually reported in technical journals, which can be hard for the average person to read and understand.
Statistical Programmer:Yes, exactly. Lay summaries are meant to make clinical trial results more accessible and transparent to patients, caregivers, and the general public.
Common Man: Who is responsible for writing these lay summaries?
Statistical Programmer: Usually, the trial sponsor is responsible for writing the lay summary.
However, some organizations and advocacy groups have also started to produce their own lay summaries to ensure that the information is presented in an unbiased and understandable way.
Common Man: That’s really interesting
Statistical Programmer: Yes, many clinical trial registries now require that a lay summary be included alongside the trial results.
Common Man: Do you know where I can find lay summaries for clinical trials?
Statistical Programmer: You can often find these summaries on the trial registry website or on the website of the organization that sponsored the trial.
Additionally, some patient advocacy groups and non-profit organizations also produce their own lay summaries of clinical trial results.
SECTIONS IN LAY SUMMARY FOR CLINICAL TRIALS
Statistical Programmer: Did you know that a lay summary for a clinical trial usually includes different sections?
Common Man: No, I didn’t. What sections are typically included in a lay summary?
Statistical Programmer:
1. the objective of the study
2. when and where the study took place
3. who participated in the study
4. what treatments patients received
5. whether there are plans for further studies
6. what the side effects were
7. how the study has helped patients and researchers
8. what the study results were
9. any additional information that may be relevant
Common Man: That’s a comprehensive list. Can you explain each section in more detail?
Statistical Programmer: Sure
1. The objective of the study is a brief description of what the study was designed to achieve
2. When and where the study took place provides information on the location and timeline of the study
3. Who participated in the study explains the demographics of the study participants, such as age, gender, and ethnicity
4. What treatments patients received describes the interventions that the patients received during the trial
5. The patients received different treatments depending on their medical condition and the clinical trial they were enrolled in
Common Man: Oh! I see. And what about the side effects?
Statistical Programmer: The section on side effects describes any adverse effects that patients experienced during the trial
What the study results were provides a summary of the findings of the trial, such as whether the treatment was effective in achieving the study objective
The clinical study did not focus on the side effects of the treatments. Its primary focus was to evaluate the effectiveness of study drug in the clinical trials
Common Man: how has the study helped patients and researchers?
Statistical Programmer: This section explains how the study findings may have an impact on patient care and treatment, as well as how the research may have contributed to the scientific community’s understanding of the disease or condition being studied and has helped patients by improving the accuracy and efficiency of data analysis, which can lead to better treatment options and outcomes. It has also helped researchers by providing them with a more efficient way of analysing data.
Common Man: Are there plans for further studies?
Statistical Programmer: Yes, there are plans for further studies to build on the findings of this study and explore the effectiveness of study drug in different types of clinical trials.
Common Man: And what about the additional information section?
The additional information section may include any other details that may be relevant to understanding the study results or their implications
For example, it may include information on the trial design, the statistical analysis methods used, or any limitations or potential biases of the study.
Also, You can find some information about Study number, study synopsis, Protocol identification number, EudraCT number and ISRCTN number, details of the sponsor, Contact details for the sponsor and study reference details, etc.





