Debdutta Banerjee
Fundamentals to Improve Data Quality in Clinical Trials
The purposes behind the faith in the requirement for perfection of clinical trial data are precisely fantasies. The legitimate weaknesses in Good Clinical Practice (GCP) are only humiliating to its logically trained promoters. Current quality administration techniques, which progressively debase the significance of examinations, are depicted and their application in clinical research prescribed. The documentation of methodology to accomplish satisfactory quality levels of preliminary information is recommended to be industrialized into a Quality Declaration.
Compiling high Quality, precise and statistically solid data is the mission for each clinical trial study; and compelling data management is fundamental to clinching precise data collection, data entry, reports, and validation. As a critical phase of the clinical research process data could be changed over into a score that was substantially more exact than movement scoring works out. The methods of data collection in clinical research incorporate information that is: manually abstracted or electronically extracted from medical records, clinical examination reports, obtained from laboratory and diagnostic tests, or from different medical devices, and patient-reported documents. Every data source is related to a strategy by which the information was acquired. Regardless of whether the information is gathered specifically for an examination, or whether information gathered for different reasons the quality management of data ought to consider the data source, pre-accumulation handling, the data collection technique, and postprocessing. While these components will probably influence data quality management protocol. Ultimately, one technique does not fit at all. Utilizing a similar strategy to treat all information will overlook the errors.
Further, regulatory authorities and professional societies like Society for clinical data management (SCDM) are concerned with the reliability of the data and initially identified the following data quality dimensions for clinical research: “electronic source data and source documentation must meet the same fundamental elements of data quality (e.g., attributable, legible, contemporaneous, original, and accurate) that are expected of paper records and must comply with all applicable statutory and regulatory requirements” .
1. Fit to Purpose
After 2008, banks across the world were blindsided by the regulatory and compliance over reach by regional and national governments. Data privacy concerns, GDPR laws and more recently BREXIT show that regulatory changes are not abating. Banks will face more legal and environmental changes which can be overcome through systematic technology adoption. Banks are intensely focusing on data analytics, artificial intelligence, biometrics and all back-end interfaces. Every decision and workflow will be determined through analytics and will be effective in risk management.
2. Critical Data Points
Lets’ recall the thalidomide disaster that blistered the history of clinical trials. That was a clinical trial of thalidomide involved the distribution of more than two and a half million tablets of thalidomide to approximately 20,000 patients across the nation—amongst which, approximately 3,760 women were of childbearing age and at least, 207 of whom were pregnant! More than one thousand physicians participated in these trials, but, to our dismay, only a few tracked their patients after dispensing the drug, leading to the biggest disaster scarring the history of clinical trials.
This principal part of the clinical trial process may appear to be genuinely clear. However, issues often arise when non-critical data is gathered for additional purposes. Despite the fact, these data should meet quality benchmarks which require extensive effort to the data management team. In such condition, standard operation procedures (SOPs) may help to reduce a bit of time and effort for data managers and eventually enhance the data quality.
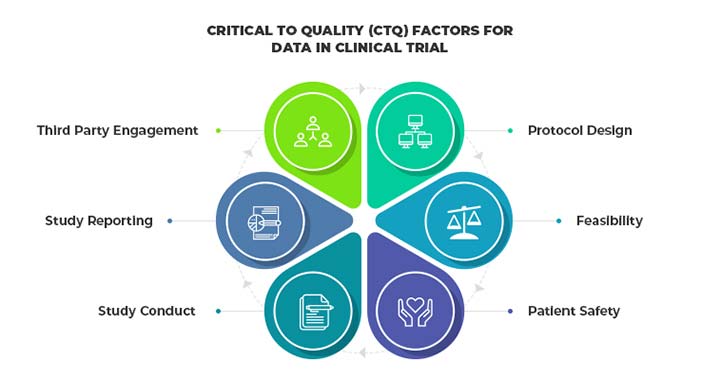
3. Data Compilation & Quality Control
SOPs are essential to successful quality control systems within the trial management & data management team. This again increases the precision of data collection by clearly plotting organizational practices and role-specific responsibilities. This specificity helps all involved stakeholders on the same page, reduces the risk for error in data collection and can make it easier to pinpoint the cause if an error occurred.
4. Data Mining for Process Optimization
Do you use your trial data to study the processor to process optimization? If not, then probably you should do. A 2017 survey of unified clinical operations found that 23% of sponsors rarely, use trial data for process optimization purpose in certain cases.
Another study, conducted in 2016, found that organizations using clinical trial data and metrics to improve trial processes have seen significant benefits. Which includes:
- Improved audit and inspection readiness
- Better visibility into performance metrics
- Cost savings
- Faster study start-up
- Shortened clinical time
5. Process Automation
Eager to enhance the activity, industry-leading organizations are deploying with robotic automation process with artificial intelligence and machine learning. The developing automated workforce is in a perfect world suited to handling numerous routine data extraction tasks that do not depend on human judgment.
Truly, robotization has turned out to be critical to reducing the expenses and complexities of clinical trials. However, it is not a panacea; rather, it’s an impetus to giving the execution measurements to enable business insight (e.g., benchmarking, prescient or estimating projections), process streamlining and efficient resource allocation, subsequently enhancing proactive arranging and separating hierarchical silos. Automation additionally enhances collaboration among stakeholders and is vital to modernizing clinical trials, streamlining communications and enabling real-time decision making.