Expert Pharmacovigilance Writing Services from SME Consulting to Comprehensive Case Management
Streamline Pharmacovigilance with Our Expert Solutions for Compliance and Safety Management
Technological advancements are transforming the pharmacovigilance (PV) market. AI-driven solutions improve efficiency, accuracy, and compliance. Machine learning and electronic health records (EHR) enhance health outcome predictions. Real-world data and advanced safety signal detection refine surveillance. Challenges remain, creating strict demand for top-tier pharmacovigilance solutions.
ACL Digital can pilot these challenges with expertise in pharmacovigilance services. Our team excels in delivering precise, compliant pharmacovigilance writing tailored to meet stringent regulatory standards. From serious adverse event reporting to aggregate report writing, our holistic pharmacovigilance platform provides comprehensive solutions that ensure your safety data is meticulously managed, enabling you to focus on innovation and patient care.

Achieve Perfection in Pharmacological with ACL Digital’s Broad-Range Services
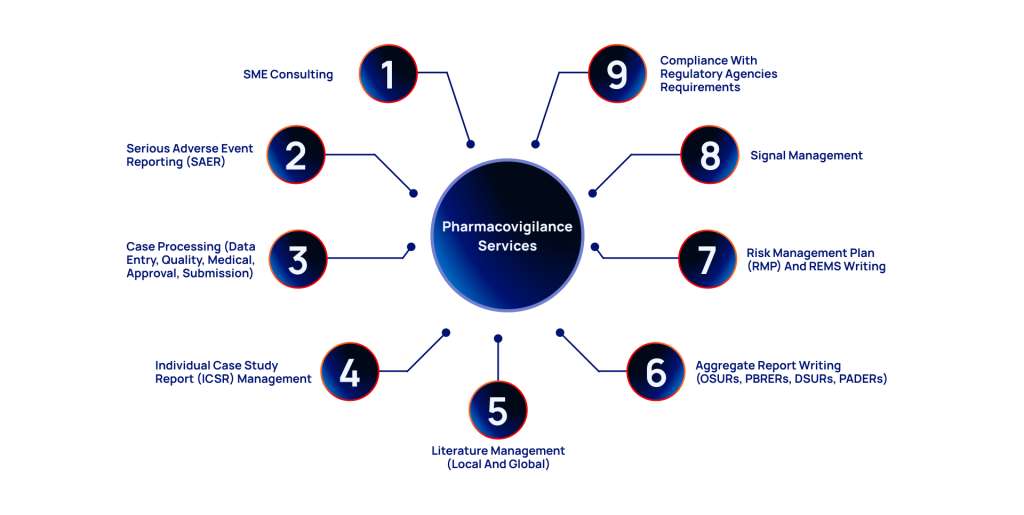
SME Consulting
Achieve desired outcomes by making your pharmacovigilance documents more meaningful and impactful. ACL Digital provides in-depth SME specializations and strategic advice.
- Tailored strategies to optimize pharmacovigilance processes
- In-depth analysis for impactful documentation
- Expert guidance on regulatory compliance
Serious Adverse Event Reporting (SAER)
Empower your SAER aspect to provide impressive clinical findings. Benefit from our expertise to meet the 100% statutory requirement and patients’ safety.
- Comprehensive reporting to meet regulatory standards
- Timely submissions to enhance patient safety
- Continuous support for quality assurance
Case Processing
Trust ACL Digital for comprehensive Case Processing services like quality assurance, streamlining data entry, medical evaluations, assurance, approvals, and submissions.
- Efficient data entry for enhanced accuracy
- Thorough quality checks to ensure compliance
- Support for medical evaluations and approvals
Individual Case Safety Report Management
We provide top-notch Individual Case Safety Reports (ICSRs) to provide accurate, timely, and consistent safety data.
- Expertise in generating reliable safety reports
- Timely updates to meet regulatory deadlines
- Focus on patient safety and data integrity
Literature Management
Extensive literature management is crucial to product and data safety. We adopt a systematic approach to help its clients remain knowledgeable or abreast of the current regulations to avoid non-compliance.
- Comprehensive monitoring of regulatory changes
- Systematic analysis to identify potential risks
- Support for informed decision-making
Aggregate Report Writing
We have a highly qualified team of writers with extensive experience generating aggregate reports within the guidelines of global regulatory authorities in preparation of PSURs, PBRERs, DSURs, and PADERs.
- Expertise in regulatory report writing
- Detailed adherence to international standards
- Quality assurance for all documentation
Risk Management Plan (RMP) and REMS writing
We prepare specific Risk Management Plans (RMPs) and REMS that balance safety issues with regulations. Our clients hire us to reduce risk while improving patient outcomes through detailed planning.
- Customized plans to mitigate risks effectively
- Focused on improving patient safety
- Compliance with regulatory expectations
Signal Management
Our expert signal management services effectively identify, assess, and mitigate potential product safety issues. Clients trust us to detect and address risks early, ensuring patient safety and regulatory compliance.
- Proactive risk assessment strategies
- Timely identification of safety signals
- Regulatory compliance assurance
Meeting Regulatory Requirements
Total regulatory compliance is crucial during pharmacovigilance documentation. We meticulously adhere to global regulatory agencies, ensuring the highest standards of regulatory scrutiny.
- Meticulous adherence to regulatory guidelines
- Continuous monitoring for compliance updates
- Commitment to the highest quality standards
Pharmacaovigilance Services
Why Choose ACL Digital’s Pharmacovigilance Services

Expert Knowledge
Our team possesses extensive knowledge of global regulatory requirements, ensuring precise compliance and minimizing risk
Tailored Solutions
We develop customized strategies to meet the specific needs of each client, ensuring alignment with organizational goals
Commitment to Safety
We prioritize patient safety in every aspect of our service delivery, ensuring that safety data is meticulously managed

Client Impact

Enhancing Clinical Data Management for Accelerated Decision-Making and Compliance
ACL Digital streamlined clinical data processes, improving efficiency and compliance while enabling real-time insights for enhanced decision-making.
The Challenges
- Disjointed data management processes leading to inefficiencies.
- Compliance issues with regulatory standards for clinical data.
- Delayed reporting due to manual data handling and analysis.
The Outcomes
- Implemented automated data management, reducing processing time by 40%.
- Ensured 100% compliance with industry regulations.
- Enabled real-time insights, enhancing decision-making speed and accuracy.
- Improved operational efficiency, saving 30% in resource costs.

Accelerating Clinical Trial Processes with ACL Digital's Cutting-Edge Solutions
ACL Digital streamlined clinical trial operations for a global CRO, improving data quality, regulatory submissions, and operational efficiency through innovative technologies.
The Challenges
- Complex trial sponsor requirements with IXRS and EDC frameworks.
- Inefficient project management due to data quality issues.
The Outcomes
- Enhanced regulatory processes with real-time trial insights.
- Improved staff productivity and efficient budget management.
- On-time submissions with optimized operational workflows.
