Ensure Global Compliance for Your Medical Devices with ACL Digital’s Expert Regulatory Affair Services
Navigate Regulatory Complexities with Confidence Through Our Support and Tailored Solutions
Understanding and adhering to life science’s regulatory affairs can be overwhelming for pharma companies. With constantly evolving requirements, vast regional requirements, and lengthy approval processes, bringing clinical products to market is filled with many roadblocks. This calls for third-party pharmaceutical regulatory affair service providers
ACL Digital Life Sciences understands these challenges and offers a wide range of innovative and advanced regulatory strategy consulting solutions to combat them. Our team of professionals can craft personalized regulatory strategies tailored to diagnostic tools and biological products. From early interactions with Health Authorities to seamless regulatory filings, we provide support to ensure timely approvals and successful market entry.

ACL Digital’s Advanced Regulatory Affairs Solutions for Industry Dominance and Competitive Advantage
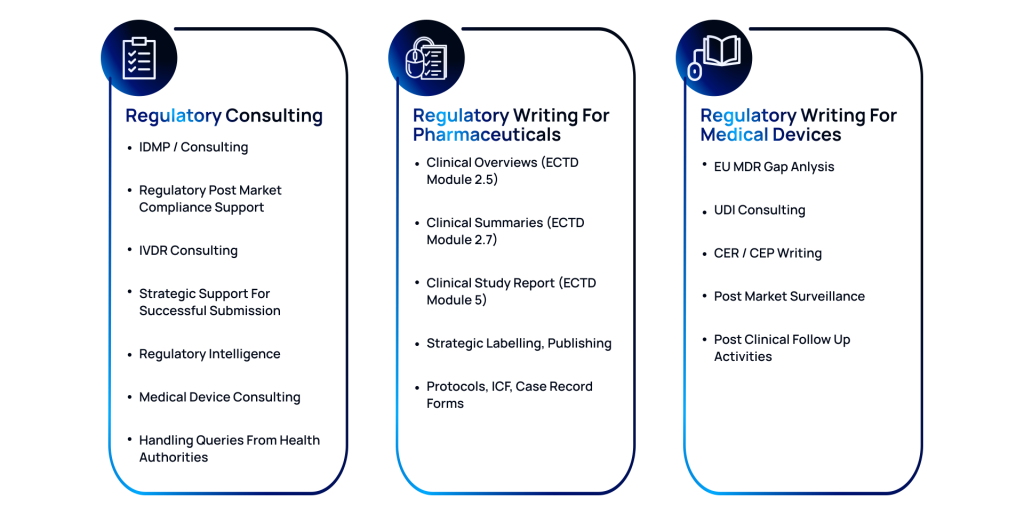
Regulatory Consulting
Achieve statutory success with our expert regulatory consulting services, specializing in IDMP and IVDR. We offer practical and precise post-market compliance support and strategic guidance for clinical submissions.
- Expert guidance on regulatory frameworks
- Timely updates on regulatory intelligence
- Support for Health Authority queries
Regulatory Writing for Pharmaceuticals
We offer expert regulatory writing services for the pharmaceutical industry to help you submit statutory-complaint clinical documentation across critical eCTD modules. Our services range from developing Clinical Overviews (Module 2.5), Clinical Summaries (Module 2.7), and in-depth Clinical Study Reports (Module 5).
- Comprehensive development of eCTD modules
- Strategic support for regulatory labeling
- Strict adherence to regulatory standards
Regulatory Writing for Medical Devices
At ACL Digital, we specialize in regulatory writing for medical devices, ensuring your products meet global standards. We conduct thorough EU MDR Gap Analyses to pinpoint and resolve regulatory issues. Our UDI consulting guarantees accurate device identification and compliance.
- Thorough analysis to identify regulatory issues
- Expert crafting of Clinical Evaluation Plans
- Support for Post-Market Surveillance activities
Regulatory Consulting & Compliance
Why Choose ACL Digital’s Regulatory Affairs Services

Deep Regulatory Expertise
Comprehensive Service Offerings
Global Reach
Client-Centric Approach
Proactive Risk Management
Data-Driven Decisions

